Glyphosate residue in honey and impacts on Africanized bee hives under field conditions
DOI:
https://doi.org/10.58951/dataset.v1i1.11Keywords:
Roundup®, contamination, Apis mellifera, colony, survival, pesticidesAbstract
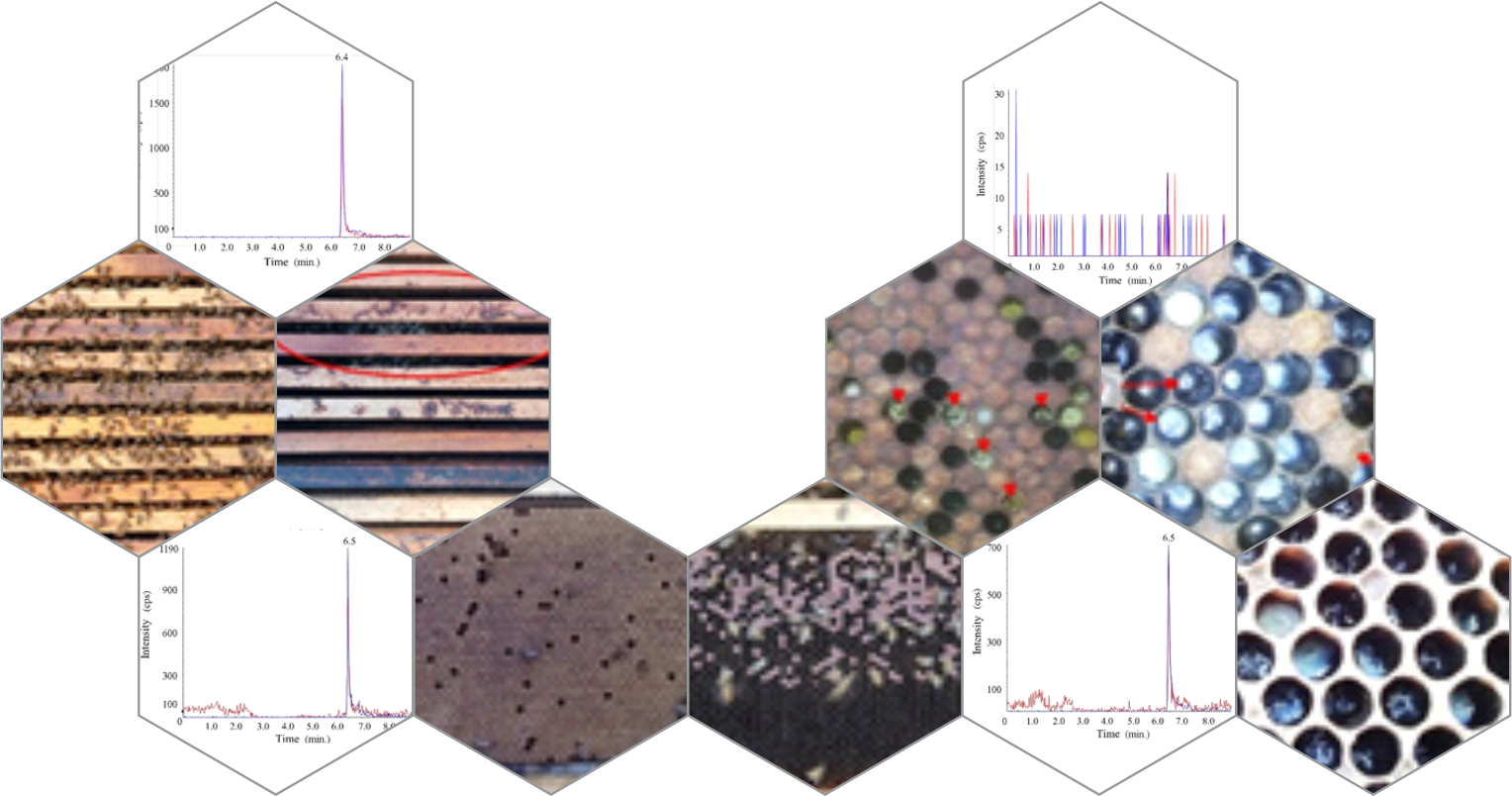
Honey and other bee products may contain residues of different substances, including pesticides, which is considered a public health problem. In addition, they characterize risks to the health of Apis mellifera, which have been showing an increasing decline in their populations. There are many protocols for identifying pesticides in bee products which, in general, are complex matrices whose results of routine investigations in control laboratories are rarely disclosed. In this sense, the objective of the present study was to determine the presence of residues of glyphosate and its metabolite aminomethylphosphonic acid (AMPA) in honey, as well as its effect on the strength of the hive of A. mellifera. Samples were collected from hives experimentally exposed to food containing a sublethal dose of Roundup® and conducted by hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry (HILIC-MS/MS). The AMPA content was lower than the method’s detection limit in honey samples from hives that received food containing the herbicide. It was possible to quantify glyphosate one week after the last artificial feeding (R1 8.45 ± 1.09 µg g−1; R2 8.15 ± 2.14 µg g−1; R3 23.90 ± 2.95 µg g−1). In a hive sample fed for more than four weeks, glyphosate was present in lower concentrations (3.12 ± 0.89 µg g−1) with no detection of AMPA. From the analysis of the strength of the hives, we observed a decrease in the population of adult individuals and the brood area, the absence of a queen, and no construction of royal cells by the workers in the hives of the Roundup® treatment in comparison to the control group, in which the hives remained with queen size, high adult and brood population, and food stock. Although present, glyphosate did not undergo degradation in honey during the evaluated period. Thus, we could infer that the presence of Roundup® in bee feed may be present in honey, representing a risk to consumers’ health and economic damage to beekeepers. This is the first long term study that evaluated the effect on hive strength of glyphosate herbicide-based residues present in pollen offered to honeybees, contributing to the understanding of the Roundup® mode of action in different aspects that affect the survival of colonies under field conditions.
References
Al-Waili, N., Salom, K., Al-Ghamdi, A., & Ansari, M. J. (2012). Antibiotic, pesticide, and microbial contaminants of honey: human health hazards. The Scientific World Journal, 2012(Table 1), 1–9. https://doi.org/10.1100/2012/930849
Almasri, H., Liberti, J., Brunet, J. L., Engel, P., & Belzunces, L. P. (2022). Mild chronic exposure to pesticides alters physiological markers of honey bee health without perturbing the core gut microbiota. Scientific Reports, 12(1), 1–15. https://doi.org/10.1038/s41598-022-08009-2
Anastassiades, M., Kolberg, D. I., Barth, A., Benkenstein, E., Dörk, D., Eichhorn, E., Zechmann, S., Mack, D., Wildgrube, C., & Sigalov, I. (2016). Quick method for the analysis of numerous highly polar pesticides in foods of plant origin via LC-MS / MS involving simultaneous extraction with methanol (QuPPe-Method) Content quoise. 2(October), 1–68.
Benbrook, C. M. (2016). Trends in glyphosate herbicide use in the United States and globally. Environmental Sciences Europe, 28(1), 1–15. https://doi.org/10.1186/s12302-016-0070-0
Berg, C. J., Peter King, H., Delenstarr, G., Kumar, R., Rubio, F., & Glaze, T. (2018). Glyphosate residue concentrations in honey attributed through geospatial analysis to proximity of large-scale agriculture and transfer off-site by bees. PLoS ONE, 13(7), 1–18. https://doi.org/10.1371/journal.pone.0198876
Bergero, M., Bosco, L., Giacomelli, A., Angelozzi, G., Perugini, M., & Merola, C. (2021). Agrochemical contamination of honey and bee bread collected in the piedmont region, Italy. Environments - MDPI, 8(7), 1–10. https://doi.org/10.3390/environments8070062
Blot, N., Veillat, L., Rouzé, R., & Delatte, H. (2019). Glyphosate, but not its metabolite AMPA, alters the honeybee gut microbiota. PLoS ONE, 14(4), 1–16. https://doi.org/10.1371/journal.pone.0215466
Cadore, A., Faita, M., Pereira, E., Bento, G., Lima, V., Steiner, J., & Poltronieri, A. (2022). Influência do uso e da cobertura do solo sobre a diversidade e a riqueza de abelhas na Ilha de Santa Catarina. Acta Biológica Catarinense, 9(2), 69–80. https://doi.org/10.21726/abc.v9i2.1596
Calatayud-Vernich, P., Calatayud, F., Simó, E., Pascual Aguilar, J. A., & Picó, Y. (2019). A two-year monitoring of pesticide hazard in-hive: High honey bee mortality rates during insecticide poisoning episodes in apiaries located near agricultural settings. Chemosphere, 232, 471–480. https://doi.org/10.1016/j.chemosphere.2019.05.170
Calatayud-Vernich, P., Calatayud, F., Simó, E., & Picó, Y. (2018). Pesticide residues in honey bees, pollen and beeswax: Assessing beehive exposure. Environmental Pollution, 241, 106–114. https://doi.org/10.1016/j.envpol.2018.05.062
Cardozo, M. M. Impactos da farinha de soja geneticamente modificada (Intacta RR2 PRO®) e do herbicida Roundup® sobre colmeias de Apis mellifera L. Master Dissertation - Universidade Federal de Santa Catarina, Centro de Ciências Agrárias, Programa de Pós-Graduação em Recursos Genéticos Vegetais, Florianópolis, 2017. 143 p.
Chaves, A., Faita, M. R., Ferreira, B. L., Poltronieri, A. S., & Nodari, R. O. (2020). Effects of glyphosate-based herbicide on royal jelly production of Apis mellifera (Hymenoptera: Apidae) in field conditions. Journal of Apicultural Research, 0(0), 1–3. https://doi.org/10.1080/00218839.2020.1844463
Chaves, A., Faita, M. R., & Nodari, R. O. (2023). Effects of fungicides on the ultrastructure of the hypopharyngeal glands and the strength of the hives of Apis mellifera Linnaeus, 1758 (Hymenoptera: Apidae). Toxicology and Applied Pharmacology, 459, 116340. https://doi.org/10.1016/J.TAAP.2022.116340
Claudianos, C., Ranson, H., Johnson, R. M., Biswas, S., Schuler, M. A., Berenbaum, M. R., Feyereisen, R., & Oakeshott, J. G. (2006). A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Molecular Biology, 15(5), 615–636. https://doi.org/10.1111/j.1365-2583.2006.00672.x
Costa, R. C., & Cruz-Landim, C. Da. (1999). Occurrence and morphometry of the hypopharyngeal glands in Scaptotrigona postica Lat. (Hymenoptera, Apidae, Meliponinae). Bioscience Journal, 24(1), 97–102.
Dallegrave, E., Mantese, F. D., Oliveira, R. T., Andrade, A. J. M., Dalsenter, P. R., & Langeloh, A. (2007). Pre- and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Archives of Toxicology, 81(9), 665–673. https://doi.org/10.1007/s00204-006-0170-5
de Souza, A. P. F., Rodrigues, N. R., & Reyes, F. G. R. (2020). Glyphosate and aminomethylphosphonic acid (AMPA) residues in Brazilian honey. Food Additives and Contaminants: Part B Surveillance, 00(00), 1–8. https://doi.org/10.1080/19393210.2020.1855676
Delaplane, K. S., Van Der Steen, J., & Guzman-Novoa, E. (2013). Standard methods for estimating strength parameters of Apis mellifera colonies. Journal of Apicultural Research, 52(1). https://doi.org/10.3896/IBRA.1.52.1.03
El Agrebi, N., Tosi, S., Wilmart, O., Scippo, M. L., de Graaf, D. C., & Saegerman, C. (2020). Honeybee and consumer’s exposure and risk characterisation to glyphosate-based herbicide (GBH) and its degradation product (AMPA): Residues in beebread, wax, and honey. Science of the Total Environment, 704, 135312. https://doi.org/10.1016/j.scitotenv.2019.135312
Evans, J. D., Aronstein, K., Chen, Y. P., Hetru, C., Imler, J. L., Jiang, H., Kanost, M., Thompson, G. J., Zou, Z., & Hultmark, D. (2006). Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Molecular Biology, 15(5), 645–656. https://doi.org/10.1111/j.1365-2583.2006.00682.x
Faita, M. R., Cardozo, M. M., Amandio, D. T. T., Orth, A. I., & Nodari, R. O. (2020). Glyphosate-based herbicides and Nosema sp. microsporidia reduce honey bee (Apis mellifera L.) survivability under laboratory conditions. Journal of Apicultural Research, 60, 1–11. https://doi.org/10.1080/00218839.2020.1736782
Faita, M. R., Chaves, A., Corrêa, C. C. G., Silveira, V., & Nodari, R. O. (2022). Proteomic profiling of royal jelly produced by Apis mellifera L. exposed to food containing herbicide-based glyphosate. Chemosphere, 292, 133334. https://doi.org/10.1016/j.chemosphere.2021.133334
Faita, M. R., Oliveira, E. de M., Alves, V. V., Orth, A. I., & Nodari, R. O. (2018). Changes in hypopharyngeal glands of nurse bees (Apis mellifera) induced by pollen-containing sublethal doses of the herbicide Roundup®. Chemosphere, 211, 566–572. https://doi.org/10.1016/j.chemosphere.2018.07.189
Franco, R., Li, S., Rodriguez-Rocha, H., Burns, M., & Panayiotidis, M. I. (2010). Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chemico-Biological Interactions, 188(2), 289–300. https://doi.org/10.1016/j.cbi.2010.06.003
Gérez, N., Pérez-Parada, A., Cesio, M. V., & Heinzen, H. (2017). Occurrence of pesticide residues in candies containing bee products. Food Control, 72, 293–299. https://doi.org/10.1016/j.foodcont.2015.10.006
Giesy, J. P., Dobson, S., & Solomon, K. R. (2000). Ecotoxicological risk assessment for Roundup® herbicide. Reviews of Environmental Contamination and Toxicology, 167, 35–120. https://doi.org/10.1007/978-1-4612-1156-3_2
Goulson, D., Nicholls, E., Botias, C., & Rotheray, E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science, 347(6229), 1255957–1255957. https://doi.org/10.1126/science.1255957
Green, J. M. (2014). Current state of herbicides in herbicide-resistant crops. Pest Management Science, 70(9), 1351–1357. https://doi.org/10.1002/ps.3727
Herbert, L. T., Vázquez, D. E., Arenas, A., & Farina, W. M. (2014). Effects of field-realistic doses of glyphosate on honeybee appetitive behaviour. Journal of Experimental Biology, 217(19), 3457–3464. https://doi.org/10.1242/jeb.109520
Huang, Z. Y., Otis, G. W., & Teal, P. E. A. (1989). Nature of brood signal activating the protein-synthesis of hypopharyngeal gland in honey bees, Apis mellifera (Apidae, Hymenoptera). Apidologie, 20(6), 455–464. https://doi.org/10.1051/apido:19890601
Karise, R., Raimets, R., Bartkevics, V., Pugajeva, I., Pihlik, P., Keres, I., Williams, I. H., Viinalass, H., & Mänd, M. (2017). Are pesticide residues in honey related to oilseed rape treatments? Chemosphere, 188, 389–396. https://doi.org/10.1016/j.chemosphere.2017.09.013
Kiljanek, T., Niewiadowska, A., Gaweł, M., Semeniuk, S., Borzęcka, M., Posyniak, A., & Pohorecka, K. (2017). Multiple pesticide residues in live and poisoned honeybees – Preliminary exposure assessment. Chemosphere, 175, 36–44. https://doi.org/10.1016/j.chemosphere.2017.02.028
Kiljanek, T., Niewiadowska, A., Semeniuk, S., Gaweł, M., Borzęcka, M., & Posyniak, A. (2016). Multi-residue method for the determination of pesticides and pesticide metabolites in honeybees by liquid and gas chromatography coupled with tandem mass spectrometry—Honeybee poisoning incidents. Journal of Chromatography A, 1435, 100–114. https://doi.org/10.1016/j.chroma.2016.01.045
Liao, L.-H., Wu, W.-Y., & Berenbaum, M. R. (2017). Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food. Scientific Reports, 7(1), 15924. https://doi.org/10.1038/s41598-017-15066-5
Matin, G., Kargar, N., & Buyukisik, H. B. (2016). Bio-monitoring of cadmium, lead, arsenic and mercury in industrial districts of Izmir, Turkey by using honey bees, propolis and pine tree leaves. Ecological Engineering, 90, 331–335. https://doi.org/10.1016/j.ecoleng.2016.01.035
Medina-Pastor, P., & Triacchini, G. (2020). The 2018 European Union report on pesticide residues in food. EFSA Journal, 18(4), 1–103. https://doi.org/10.2903/j.efsa.2020.6057
Mesnage, R., Bernay, B., & Séralini, G. E. (2012). Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology, 314(2–3), 122–128. https://doi.org/10.1016/j.tox.2012.09.006
Mitchell, E. A. D., Mulhauser, B., Mulot, M., Mutabazi, A., Glauser, G., & Aebi, A. (2017). A worldwide survey of neonicotinoids in honey. Science, 358(6359). https://doi.org/10.1126/science.aan3684
Moritz, R. F. A., & Fuchs, S. (1998). Organization of honeybee colonies: Characteristics and consequences of a superorganism concept. Apidologie, 29(1–2), 7–21. https://doi.org/10.1051/apido:19980101
Motta, E. V. S., Mak, M., De Jong, T. K., Powell, J. E., O’Donnell, A., Suhr, K. J., Riddington, I. M., & Moran, N. A. (2020). Oral or topical exposure to glyphosate in herbicide formulation impacts the gut microbiota and survival rates of honey bees. Applied and Environmental Microbiology, 86(18). https://doi.org/10.1128/AEM.01150-20
Motta, E. V. S., Raymann, K., & Moran, N. A. (2018). Glyphosate perturbs the gut microbiota of honey bees. Proceedings of the National Academy of Sciences, 115(41), 10305–10310. https://doi.org/10.1073/pnas.1803880115
Mullin, C. A., Fine, J. D., Reynolds, R. D., & Frazier, M. T. (2016). Toxicological risks of agrochemical spray adjuvants: organosilicone surfactants may not be safe. Frontiers in Public Health, 4(92), 1–8. https://doi.org/10.3389/fpubh.2016.00092
Mullin, C. A., Frazier, M., Frazier, J. L., Ashcraft, S., & Simonds, R. (2010). high levels of miticides and agrochemicals in North American apiaries : implications for honey bee health. PLoS ONE, 5(3). https://doi.org/10.1371/journal.pone.0009754
Odemer, R., Alkassab, A. T., Bischoff, G., Frommberger, M., Wernecke, A., Wirtz, I. P., ... & Odemer, F. (2020). Chronic high glyphosate exposure delays individual worker bee (Apis mellifera L.) development under field conditions. Insects, 11(10), 664. https://doi.org/10.3390/insects11100664
Othman, N. H. (2012). Honey and cancer: Sustainable inverse relationship particularly for developing nations-a review. Evidence-Based Complementary and Alternative Medicine, 2012. https://doi.org/10.1155/2012/410406
Pacífico da Silva, I., Oliveira, F. A. S., Pedroza, H. P., Gadelha, I. C. N., Melo, M. M., & Soto-Blanco, B. (2015). Pesticide exposure of honeybees (Apis mellifera) pollinating melon crops. Apidologie, 46(6), 703–715. https://doi.org/10.1007/s13592-015-0360-3
Pareja, L., Jesús, F., Heinzen, H., Hernando, M. D., Rajski, Ł., & Fernández-Alba, A. R. (2019). Evaluation of glyphosate and AMPA in honey by water extraction followed by ion chromatography mass spectrometry. A pilot monitoring study. Analytical Methods, 11(16), 2123–2128. https://doi.org/10.1039/c9ay00543a
Parrón, T., Requena, M., Hernández, A. F., & Alarcón, R. (2011). Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicology and Applied Pharmacology, 256(3), 379–385. https://doi.org/10.1016/j.taap.2011.05.006
Pettis, J. S., Lichtenberg, E. M., Andree, M., Stitzinger, J., Rose, R., & VanEngelsdorp, D. (2013). crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE, 8(7), e70182. https://doi.org/10.1371/journal.pone.0070182
PPDB (Pesticide Properties DataBase), 2022. Glyphosate (Ref: Mon 0573). University of Hertfordshire. Available at: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/373.htm. Accessed on December 20, 2022).
Raimets, R., Bontšutšnaja, A., Bartkevics, V., Pugajeva, I., Kaart, T., Puusepp, L., Pihlik, P., Keres, I., Viinalass, H., Mänd, M., & Karise, R. (2020). Pesticide residues in beehive matrices are dependent on collection time and matrix type but independent of proportion of foraged oilseed rape and agricultural land in foraging territory. Chemosphere, 238(August 2019). https://doi.org/10.1016/j.chemosphere.2019.124555
Romano, R. M., Romano, M. A., Bernardi, M. M., Furtado, P. V., & Oliveira, C. A. (2010). Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Archives of Toxicology, 84(4), 309–317. https://doi.org/10.1007/s00204-009-0494-z
Rondeau, G., Sánchez-Bayo, F., Tennekes, H. A., Decourtye, A., Ramírez-Romero, R., & Desneux, N. (2014). Delayed and time-cumulative toxicity of imidacloprid in bees, ants and termites. Scientific Reports, 4, 1–8. https://doi.org/10.1038/srep05566
Rubio, F., Guo, E., & Kamp, L. (2014). Survey of glyphosate residues in honey, corn and soy products. Journal of Environmental & Analytical Toxicology, 05(01), 1–8. https://doi.org/10.4172/2161-0525.1000249
Rundlöf, M., Andersson, G. K. S., Bommarco, R., Fries, I., Hederström, V., Herbertsson, L., Jonsson, O., Klatt, B. K., Pedersen, T. R., Yourstone, J., & Smith, H. G. (2015). Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature, 521(7550), 77–80. https://doi.org/10.1038/nature14420
Sanchez-Bayo, F., & Goka, K. (2014). Pesticide residues and bees - A risk assessment. PLoS ONE, 9(4). https://doi.org/10.1371/journal.pone.0094482
Sánchez-Bayo, F., Goulson, D., Pennacchio, F., Nazzi, F., Goka, K., & Desneux, N. (2016). Are bee diseases linked to pesticides? - A brief review. Environment International 89–90, 7-11. https://doi.org/10.1016/j.envint.2016.01.009
Saurat, D., Raffy, G., Bonvallot, N., Monfort, C., Fardel, O., Glorennec, P., Chevrier, C., & Le Bot, B. (2022). Determination of glyphosate and AMPA in indoor settled dust by hydrophilic interaction liquid chromatography with tandem mass spectrometry and implications for human exposure. Journal of Hazardous Materials, 130654. https://doi.org/10.1016/J.JHAZMAT.2022.130654
Spurgeon, D., Hesketh, H., Lahive, E., Svendsen, C., Baas, J., Robinson, A., Horton, A., & Heard, M. (2016). Chronic oral lethal and sub-lethal toxicities of different binary mixtures of pesticides and contaminants in bees (Apis mellifera, Osmia bicornis and Bombus terrestris) Centre for Ecology & Hydrology. EFSA Supporting Publications, 13(9). https://doi.org/10.2903/sp.efsa.2016.EN-1076
Thompson, H. M., Levine, S. L., Doering, J., Norman, S., Manson, P., Sutton, P., & von Mérey, G. (2014). Evaluating exposure and potential effects on honeybee brood (Apis mellifera) development using glyphosate as an example. Integrated Environmental Assessment and Management, 10(3), 463–470. https://doi.org/10.1002/ieam.1529
Thompson, T. S., van den Heever, J. P., & Limanowka, R. E. (2019). Determination of glyphosate, AMPA, and glufosinate in honey by online solid-phase extraction-liquid chromatography-tandem mass spectrometry. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 36(3), 434–446. https://doi.org/10.1080/19440049.2019.1577993
Traynor, K. S., Pettis, J. S., Tarpy, D. R., Mullin, C. A., Frazier, J. L., Frazier, M., & vanEngelsdorp, D. (2016). In-hive pesticide exposome: assessing risks to migratory honey bees from in-hive pesticide contamination in the Eastern United States. Scientific Reports. https://doi.org/10.1038/srep33207
Tsvetkov, N., Sood, K., Patel, H. S., Malena, D. A., Gajiwala, P. H., Maciukiewicz, P., Fournier, V., & Zayed, A. (2017). Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science, 356(6345), 1395–1397. https://doi.org/10.1126/science.aam7470
Wehling, M., Ohe, W. V, Brasse, D., Forster, R., Oomen, P. A., & Thompson, H. M. (2009). Colony losses - interactions of plant protection products and other factors. Julius-Kühn-Archiv, 423, 153–154.
Wiest, L., Buleté, A., Giroud, B., Fratta, C., Amic, S., Lambert, O., Pouliquen, H., & Arnaudguilhem, C. (2011). Multi-residue analysis of 80 environmental contaminants in honeys , honeybees and pollens by one extraction procedure followed by liquid and gas chromatography coupled with mass spectrometric detection. Journal of Chromatography A, 1218(34), 5743–5756. https://doi.org/10.1016/j.chroma.2011.06.079
Woodcock, B. A., Bullock, J. M., Shore, R. F., Heard, M. S., Pereira, M. G., Redhead, J., Ridding, L., Dean, H., Sleep, D., Henrys, P., Peyton, J., Hulmes, S., Hulmes, L., Sárospataki, M., Saure, C., Edwards, M., Genersch, E., Knäbe, S., & Pywell, R. F. (2017). Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science, 356(6345), 1393–1395. https://doi.org/10.1126/science.aaa1190
Yoshioka, N., Asano, M., Kuse, A., Mitsuhashi, T., Nagasaki, Y., & Ueno, Y. (2011). Rapid determination of glyphosate, glufosinate, bialaphos, and their major metabolites in serum by liquid chromatography-tandem mass spectrometry using hydrophilic interaction chromatography. Journal of Chromatography A, 1218(23), 3675–3680. https://doi.org/10.1016/j.chroma.2011.04.021
Zhu, Y. C., Yao, J., Adamczyk, J., & Luttrell, R. (2017). Synergistic toxicity and physiological impact of imidacloprid alone and binary mixtures with seven representative pesticides on honey bee (Apis mellifera ). PLoS ONE 12(5): e0176837. https://doi.org/10.1371/journal.pone.0176837.
Zioga, E., White, B., & Stout, J. C. (2022). Glyphosate used as desiccant contaminates plant pollen and nectar of non-target plant species. Heliyon, 8(12). https://doi.org/10.1016/j.heliyon.2022.e12179
Zoller, O., Rhyn, P., Rupp, H., Zarn, J. A., & Geiser, C. (2018). Glyphosate residues in Swiss market foods: monitoring and risk evaluation. Food Additives and Contaminants: Part B Surveillance, 11(2), 83–91. https://doi.org/10.1080/19393210.2017.1419509
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Dataset Reports

This work is licensed under a Creative Commons Attribution 4.0 International License.
This journal publishes its Open Access articles under a Creative Commons license (CC BY 4.0).
You are free to:
Share — copy and redistribute the material in any medium or format for any purpose, even commercially.
Adapt — remix, transform, and build upon the material for any purpose, even commercially.
The licensor cannot revoke these freedoms as long as you follow the license terms.
Under the following terms:
Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
Notices:
You do not have to comply with the license for elements of the material in the public domain or where your use is permitted by an applicable exception or limitation.
No warranties are given. The license may not give you all of the permissions necessary for your intended use. For example, other rights such as publicity, privacy, or moral rights may limit how you use the material.




